상품 정보
상품 기본설명
상품 상세설명
Product introduction
Octyl-4FF can interact with some hydrophobic protein or antibody under high ionic strength conditions (high ionic strength may increase the interaction between ligand and hydrophobic groups), thus to achieve the purpose of separation and purification. Butyl S-6FF is mainly used for capture of initial samples and moderate and fine purification of samples.
Advantages
1. Rapid, easy to use (one-step purification).
2. Compared with reversed-phase chromatography, the ligand concentration in the hydrophobic interaction chromatography media is low and the elution conditions are mild, which helps to maintain the biological activity of biomolecules.
3. Wide application. Octyl-4FF can be applied in preliminary capture, moderate purification and fine purification, and it can be repeatedly used in combination ion exchange chromatography media.
Performance index
Ligand | Octyl |
Matrix | Highly cross-linked 4% agarose |
Particle size range | 45-65 µm |
Average particle size | 90 µm |
Ligand density | ~5 µmol/mL |
Binding capacity | ~10 mg (Lysyme)/mL (media) |
pH stability | 2-14 (short-term) 3-13 (long-term) |
Chemical stability | All of the commonly used buffers, 1M Acetic acid, 1M NaOH, 8M urea, 6M guanidine hydrochloride, 30% Isopropyl alcohol, 70% Ethanol |
Flow rate | 300-700 cm/h (0.3 MPa, XK16/40, column bed height: 30 cm) |
Storage buffer | 20% Ethanol |
Storage temperature | 4~30℃ (4~8℃ is preferred) |
Factors affecting hydrophobic chromatography
Influence | Function mechanism | Suggestion |
Ligand structure | The binding ability between different ligands and proteins is different. | Pre-experiment is recommended to screen suitable media, which can be referred to in Figure 1. |
Ligand density | The higher the concentration of ligand, the stronger the binding ability will be. | Pre-experiment is recommended to screen the optimum ligand density. |
Sample properties | The hydrophobicity of protein depends on the distribution of hydrophobic groups on its surface. | / |
Salt concentration | The higher the salt concentration, the stronger the binding between the ligand and the protein will be, but the excessive high concentration of salt may result in protein precipitation. | Check the solubility and stability of protein under different salt concentrations. |
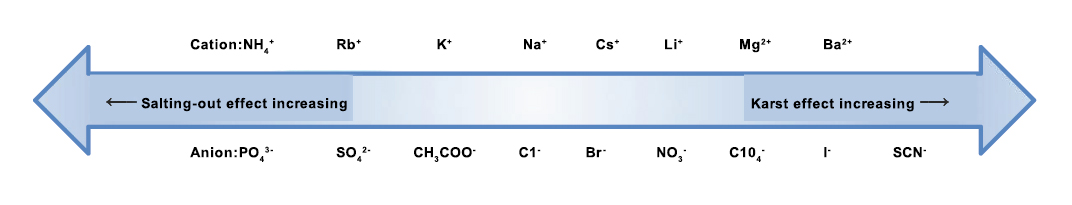
Salt type | Different kinds of salts can result in different binding effects. | (NH4)2SO4 and NaCl are preferred, other selections can refer to Figure 2. |
Temperature | The higher the temperature, the stronger the protein hydrophobic will be. | Temperature must be maintained to be the same. Room temperature is recommended. |
pH | Excessive high or low pH may affect the solubility and stability of protein, and pH can affect the binding effect. | The recommended pH range is 5.0-8.5 on the premise of ensuring the solubility and stability of protein. |
Figure 1: Hydrophobic properties comparison of ligands with different concentrations
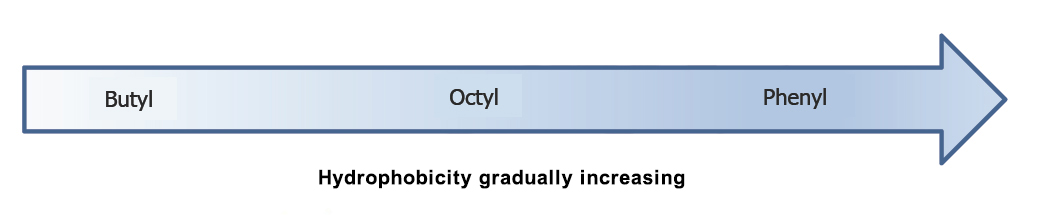
Figure 2: Salt dissolution and salting out effect of different ions
상품문의
등록된 상품문의
상품문의가 없습니다.
반품/교환정보
셀젠바이오샵에서는 다음과 같은 기간 및 내용으로 상품에 대하여 교환, 반품, 환불을 보장하고 있으며, 상품의 반환에 의한추가비용을 고객에게 부담시지키 않습니다. (단, 고객 변심 또는 주문 반복으로 인한 경우의 반환비용은 고객님이 부담하셔야
합니다.)
::: 교환 및 반품이 가능한 경우:::
단, 상품을 개봉하여 상품가치가 상실된 경우에는 교환/반품이 불가능합니다.
:::교환 및 반품이 불가능한 경우:::
주문 취소 및 반품으로 환불을 요청하실 경우에는 E-mail(celgen-bio@celgen-bio.com)이나 고객만족센터 (042-824-9026)을
통해 요청하시면 친절하게 처리해 드리겠습니다.
주문 취소 후 반품 가능 여부를 확인한 다음 3일 이내에 결제 금액을 환불해 드리겠습니다.