상품 정보
상품 기본설명
상품 상세설명
Application
This kit applies the GPO-PAP method and it can be used for in vitro determination of triglyceride (TG) content in serum, plasma, cells, culture supernatant and other samples.
Detection principle
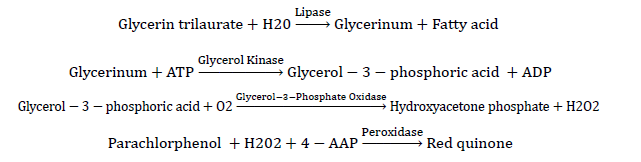
The color depth of the generated quinones is directly proportional to the triglyceride content. The absorbance values of the standard tube and the sample tube are measured respectively, and the triglyceride content in the sample can be calculated.
Experimental instrument
Test tube, Micropipettor, Vortex mixer, Water bath, Microplate reader or Biochemical analyzer (510 nm)
Sample preparation
1. Serum (Plasma): Detect the sample directly. If the concentration is beyond the linear range, then dilute the sample with normal saline before detection.
2. Culture supernatant sample: Collect the culture supernatant, centrifuge at 1000 rpm for 10 min, and take the supernatant for detection.
[Note]: It is generally recommended that the cell density should be more than 1×106/mL.
3. Tissue sample: Accurately weigh the tissue weight, add 9 times the volume of homogenate media according to the ratio of Weight (g): Volume (mL) =1:9. Mechanical homogenate the sample in ice water bath. Centrifuge at 2500 rpm for 10 min, then take the supernatant for detection.
[Note]: (1) If the tissue sample is not a high-fat sample, the homogenate media should be phosphate buffer (0.1 mol/L, pH 7.4) or normal saline.
(2) If the tissue sample is high-fat sample or partly high lipid sample, the homogenate media should be absolute alcohol.
4. Cell sample:
Cell collection: Take the prepared cell suspension and centrifuge at 1000 rpm for 10 min. Discard the supernatant and keep the cell sediment. Wash the sediment with isosmotic solution (0.1 mol/L, pH7~7.4 phosphate buffer was recommended) 1~2 times, centrifuge at 1000 rpm for 10 min. Discard the supernatant and keep the cell sediment.
Cell disruption: Add 0.2~0.3 mL of homogenate media (0.1 mol/L, pH7~7.4 phosphate buffer or normal saline was recommended). Sonicate in ice water bath (power: 300 W, 3~5 second/time, interval for 30 sec, repeat for 3~5 times) or grind with hand-operated. The prepared homogenate kept for detection without centrifugation. The cell can also be lysed with the cell lysate buffer (Triton X-100, 1~2%, 30~40 min), then take the prepared lysate for detection directly without centrifugation.
[Note]: It is generally recommended that the cell density should be more than 1×106/ml. The disrupted cell can be observed with microscope that whether the cell is broken completely.
Operation steps
Operate with 96T microplate. Colorimetric assay by microplate reader | |||
| Blank well | Standard well | Sample well |
Distilled water (μL) | 2.5 |
|
|
Standard (μL) |
| 2.5 |
|
Sample (μL) |
|
| 2.5 |
Working solution (μL) | 250 | 250 | 250 |
Mix thoroughly, incubate at 37℃ for 10 min, measure the OD value at 510 nm with microplate reader.
Operate with automatic biochemical analyzer | |
Sample volume/Water (μL) | 2.5 |
Working solution (μL) | 250 |
Incubate at 37℃ for 10 min, set zero with water+ working solution, measure the absorbance value A at 510 nm. | |
Main wavelength(nm) | 510 |
Reaction type | Endpoint method |
Reaction direction | (+) |
Performance index
1. The absorbance of blank tube is ≤ 0.200 (optical path=0.5 cm).
2. Linear range: 0~9.04 mmol/L, r2 > 0.995.
3. Sensitivity: The absorbance value (△A) is between 0.2200 ~ 0.2900 when testing 2.26 mmol/L samples.
4. Accuracy: Relative deviation ≤ 10%.
5. Repeatability: Precision ≤ 5.0%, inter-CV ≤ 8%.
6. Storage: The valid of kit is 12 months when stored at 2℃~8℃ in the dark. It is stable for 1 month when stored at 2℃~8℃ in the dark after opening.
Notes
1. The kit is for scientific research only.
2. Instructions should be followed strictly, changes of operation may result in unreliable results.
3. The validity of kit is 12 months.
4. Do not use components from different batches of kit.
5. If the sample content is beyond the maximum limit, please dilute the sample with normal saline before detection, and multiply the result by the dilution ratio.
6. Protect the reagent from contamination of glucose, cholesterol, etc.
7. The amount of reagent and sample can be increased and decreased as the ratio of 1:100 according to the requirement of automatic biochemical analyzer.
상품문의
등록된 상품문의
상품문의가 없습니다.
반품/교환정보
셀젠바이오샵에서는 다음과 같은 기간 및 내용으로 상품에 대하여 교환, 반품, 환불을 보장하고 있으며, 상품의 반환에 의한추가비용을 고객에게 부담시지키 않습니다. (단, 고객 변심 또는 주문 반복으로 인한 경우의 반환비용은 고객님이 부담하셔야
합니다.)
::: 교환 및 반품이 가능한 경우:::
단, 상품을 개봉하여 상품가치가 상실된 경우에는 교환/반품이 불가능합니다.
:::교환 및 반품이 불가능한 경우:::
주문 취소 및 반품으로 환불을 요청하실 경우에는 E-mail(celgen-bio@celgen-bio.com)이나 고객만족센터 (042-824-9026)을
통해 요청하시면 친절하게 처리해 드리겠습니다.
주문 취소 후 반품 가능 여부를 확인한 다음 3일 이내에 결제 금액을 환불해 드리겠습니다.